About
Table of contents
State of research
In clinical practice, there are various approaches for the treatment of cocaine use disorder. These are either psychotherapeutic or pharmacological in nature. However, current psychotherapeutic treatments do not achieve good results for all affected individuals, and so far, no medication has been approved for the treatment of cocaine addiction.
Previous research has shown that the development of cocaine use disorder is associated with various changes in the brain’s reward system. This leads to an increased significance of cocaine and related cues, while natural rewards - such as social interactions, good food, or physical activities - may lose their appeal. In addition, there are changes in glutamate levels, an important neurotransmitter in the brain that plays a key role in cocaine craving.
Currently, there is no treatment approach that specifically targets both of these changes in the reward system and in behavior.
Initial evidence suggests that ketamine - a medication that can influence glutamate levels - may reduce cocaine craving and use. Furthermore, there are promising findings showing that neurofeedback training - a form of brain training - can specifically increase the sensitivity of the reward system towards natural rewards. This video explains how the neurofeedback training works (starting at 11:43).
By combining these two treatment methods, we aim to determine to what extent they are effective in the treatment of cocaine use disorder. Our novel treatment approach combines a single ketamine infusion with neurofeedback training.
Study objective
The aim of this study is to determine whether targeted neurofeedback training and a single ketamine infusion can help individuals reduce their craving for cocaine and their cocaine use. In addition, the study will investigate whether the combination of these two approaches leads to an additional enhancement of effectiveness.
It is expected that a single administration of ketamine, neurofeedback training, and especially the combination of these two methods will result in an improvement of the symptoms of cocaine use disorder.
Procedure & location
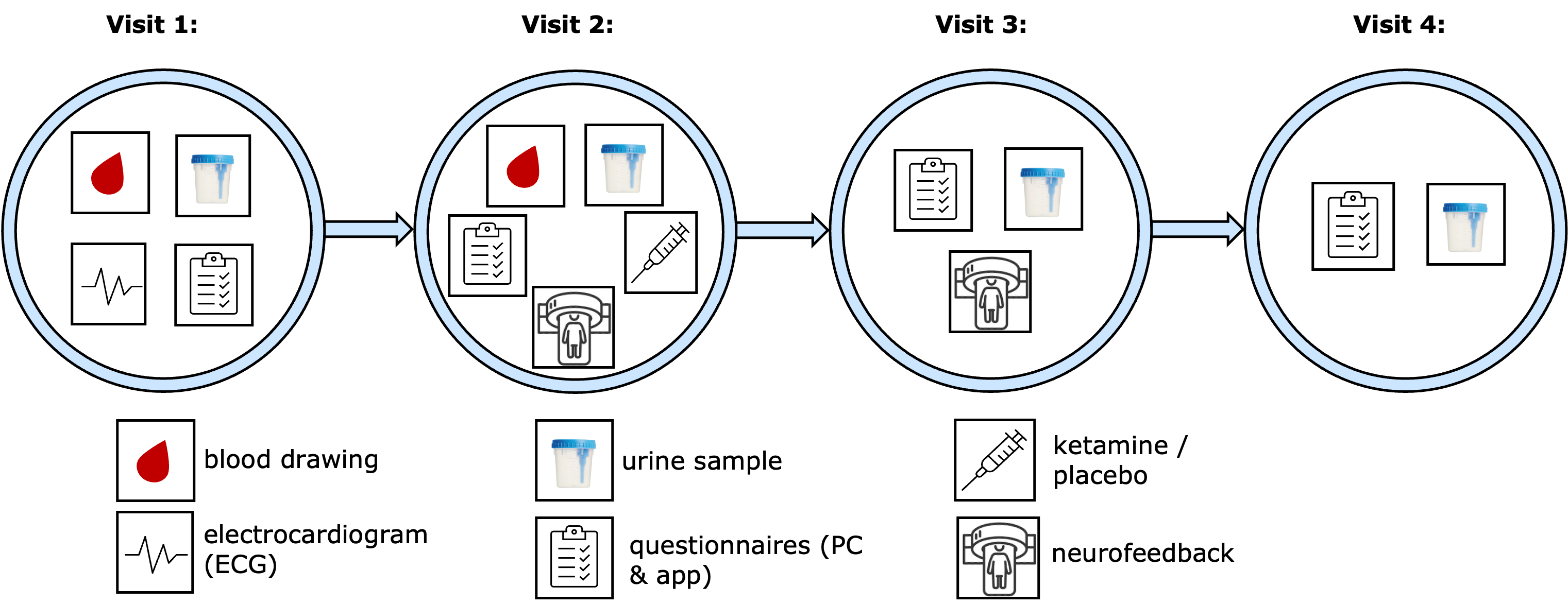
Visits 1 to 4 of the study will take place at the Psychiatric University Hospital Zurich, Lenggstrasse 31, 8032 Zurich. Appointments 5 and 6 can be attended from home. The six appointments are scheduled over a period of six months.
The first visit (ca. 3.5h) ensures that all eligibility criteria are met and that the participant’s health condition allows them to take part in the study. This includes an electrocardiogram (ECG) and a brief medical examination with blood sampling. In addition, the therapeutic goals regarding cocaine use will be discussed together.
On the second visit (ca. 7h), the actual treatment takes place: participants will receive either ketamine or a placebo via infusion, in combination with either real or sham neurofeedback training. Which treatment combination a participant receives is determined randomly. Both the infusion (ketamine or placebo) and the neurofeedback training take place in a magnetic resonance imaging (MRI) scanner. This allows for the investigation of both glutamate levels and the sensitivity to natural reward stimuli within the reward system. A urine sample will be used to check for the use of other substances. A blood sample will be taken to examine markers of neuroplasticity.
One week later, the third visit (ca. 4h) takes place, during which the neurofeedback training is repeated.
The fourth visit (ca. 1.5h) occurs approximately one month after the third visit. Here, participants will be asked about their current substance use behavior.
At all on-site visits, participants will be asked to provide an urine sample so that current cocaine use can be assessed.
The fifth and sixth visits (ca. 20 min and 15 min) can be completed from home. Participants will fill out questionnaires about their substance use behaviorand well-being.
The duration of each visit is shown in Figure 1.
Eligibility criteria
Individuals seeking to change their cocaine use patterns may participate in our study if the following criteria are met:
- Age between 18 and 55 years
- Regular cocaine use
- Good knowledge of German or English
Participation is not possible if, among other things, any of the following apply:
- Bipolar or psychotic disorder
- Severe substance use disorder (other than cocaine and nicotine)
- Untreated high blood pressure
- Untreated hyperthyroidism
- Increased intracranial pressure
- Pregnant or breastfeeding women
- Allergy or sensitivity to ketamine
- Current participation in another clinical study
Benefits & risks
Our study offers the opportunity to use a novel treatment approach. While there is no guaranteed personal benefit from participating, there is evidence suggesting that both ketamine treatment and neurofeedback training may have positive effects on cocaine use disorder.
The risks of participation include possible side effects of ketamine; however, ketamine is a safe and well-studied medication that has already been widely used for other psychiatric disorders such as depression.
For full study participation, participants will receive CHF 370.-.